How to search the DB¶
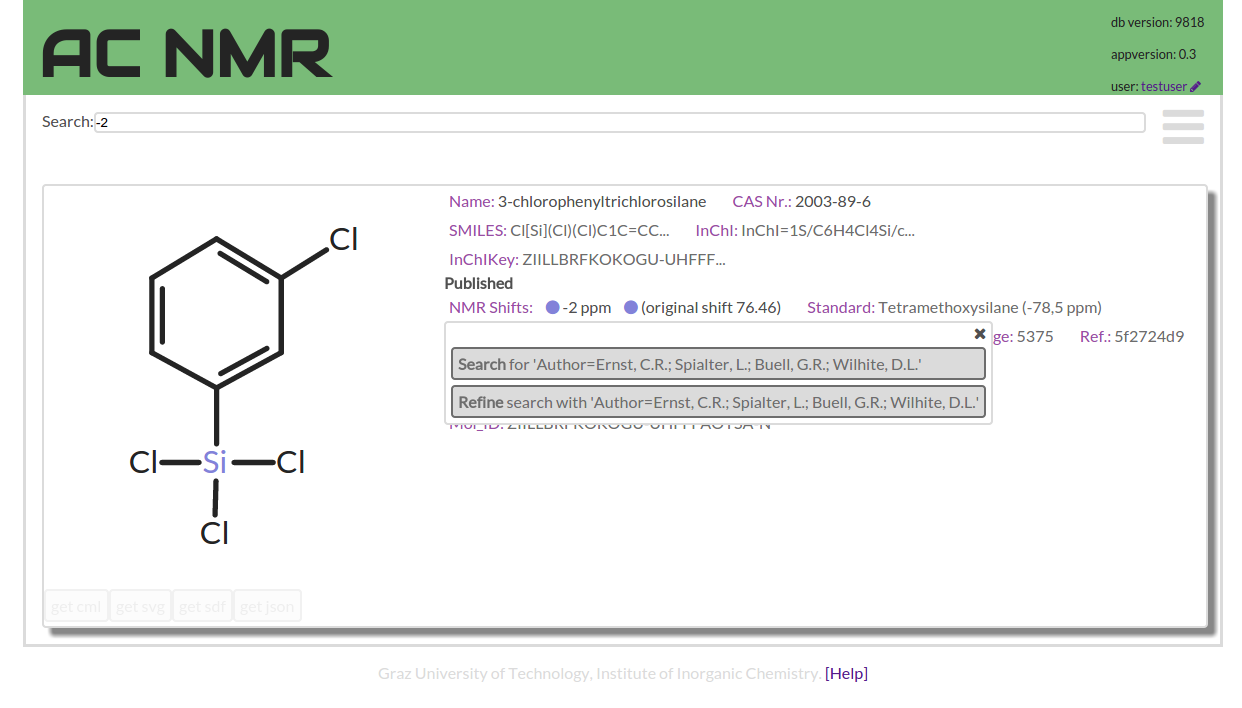
The most important interface for all searching is the central search field which is almost always visible.
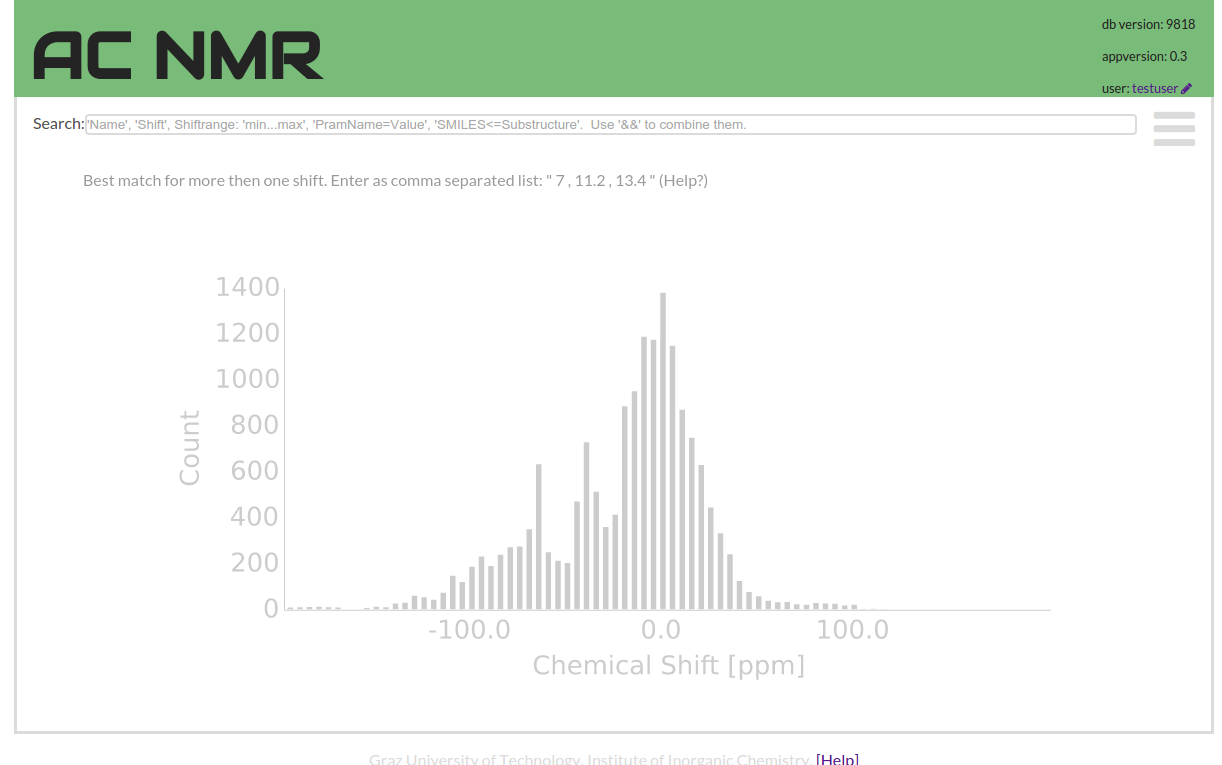
The search field tries to do the most intuitive thing for substring and shift searches. But also it provides a simple syntax to compose more complex query combinations.
If you enter a number value into the search field you find the compounds with a shift close to this number. If you enter a word or words the search performs a substring search.
This substring search is quite stubborn as it can only find exact matches. In order to help the user entering a successful query the input field will complete user input by offering suitable words that occur in the data. This substring search, searches compound names, publication authors and titles, also InChI codes or data-document IDs.
Detailed Search¶
The other user interface element designed to assist with search is the search detail form. It is accessible through the symbol with the three horizontal bars next to the search field.
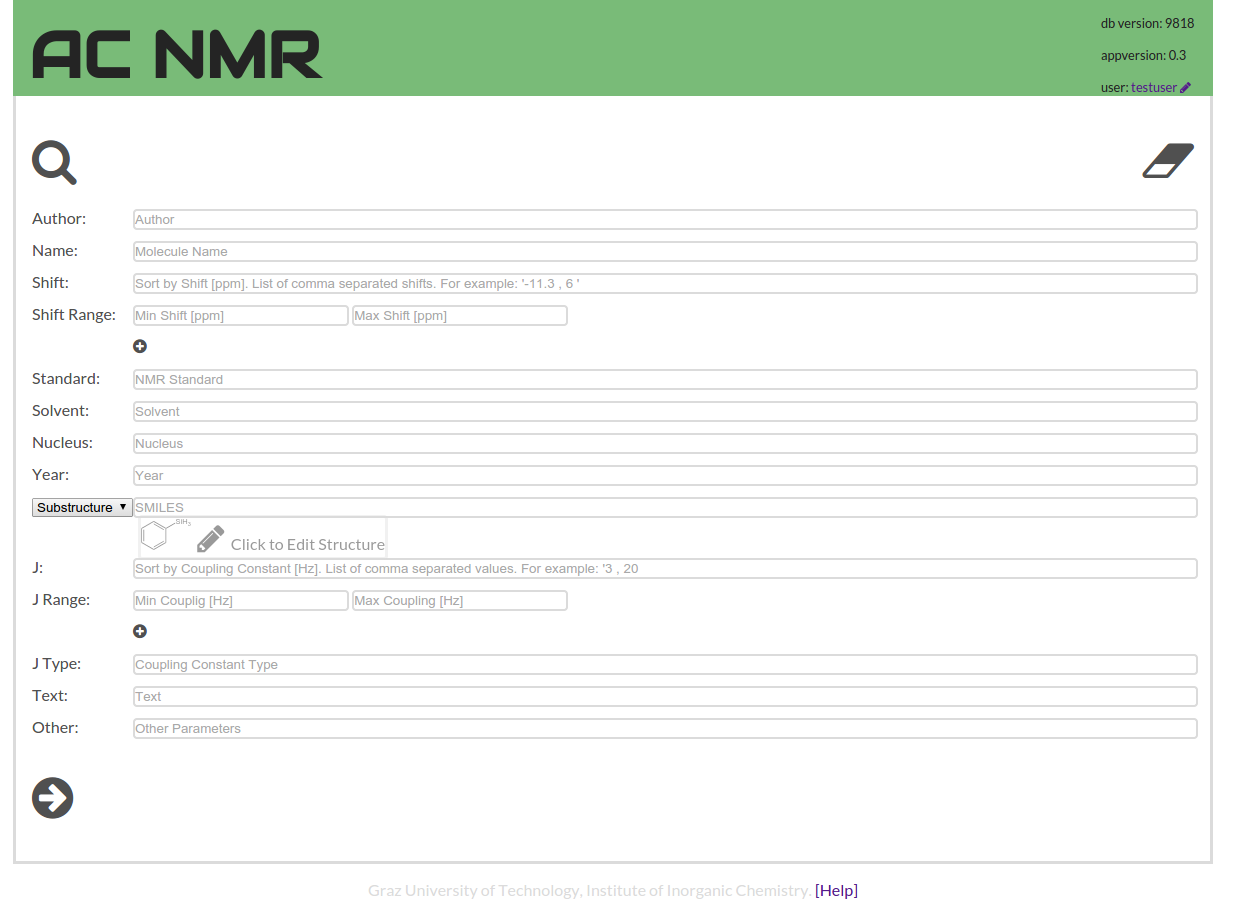
If you already had a query in the search field, the input is not erased but decomposed into the search components and the form is filled with the values. In the other direction, if you have entered a set of parameters into the detailed search form, and you proceed to the searching, the parameters get concatenated into a single query string. Which reflects one of the design principles of the ACNMR data base that the query producing the results is always visible, and part of the URL.
In this form you find a button to edit a query structure for substructure or similarity search.
Combined Search¶
For combining two or more conditions the “&&” operator may be used to do this.
-2.3 ... -1.2 && 10.0 ... 13.4
This combines two shift-range searches, such that it returns only compounds that have shifts in both ranges.
You can combine shift searches like that:
7 && 11 && 14
This will sort the results by the sum of the smallest distances. It will however, use all available shift values of the entry, also across nucleus and solvents. It is therefore possible to refine the results by adding more constraints:
-121.3 && 4 && nucleus=Si
As an alternative syntax for shift sorting, it is possible to enter a list of shifts as comma separated list:
7.0, 11.2, 14.0
Queryparameters¶
The parameter name are case insensitive and there are a few aliases for easier use.
The special value “undefined” lets you search for fields that are not defined yet. Example: doi=undefined
Mol and Literature¶
- author=
- Substring in literature author field. Example: author=Marsmann
- boilingpoint=
- Substring in boiling point field. Example: boilingpoint=20
- CAS_RN=
- Substring in CAS_RN. Example: CAS_RN=1206-46-8
- Comment=
- Substring in comment. Example: comment=Si assignment
- DOCTYPE=
- Substring in literature type like journal, book, article. Example: DOCTYPE=article
- DOI=
- Substring in literature DOI. Example: DOI=http://dx.doi.org/10.1016/s0022-328x(00)82875-2
- Editor=
- Substring in literature editor. Example: Editor=Rappoport
- FORMULA=
- Substring in sum formular. Example: FORMULA=C74
- InChI=
- Substring in International Chemical Indentifier. Example: inchi=C74
- InChI<=valid inchi>
- Substring search. Example: InChI<=InChI=1S/C5H12Si/c1-5-6(2,3)4/h5H,1H2,2-4H3
- InChI~=valid inchi>
- Similarity search. Example: InChI~=InChI=1S/C5H12Si/c1-5-6(2,3)4/h5H,1H2,2-4H3
- InChIKey=
- Substring. Example: InChIKey=GCSJLQSCSDMKTP-UHFFFAOYSA-N
- Keyword
- Substring in literature key words. Example: keyword=silanes
- MSPeakListPeaks=
- Substring. Example: MSPeakListPeaks=1531
- MSPeakListSourceID
- Substring. Example: MSPeakListSourceID=EI MS
- MeltingPoint=
- Substring. Example: MeltingPoint=20
- Mol_ID=
- exact Mol id. Example: mol_id=DZNQKXKZINSVPH-UHFFFAOYSA-N
- Mol_Name=
- Substring in Compound Name. Example: Mol_Name=cyclopentadienyl
- Page=
- Substring literature page. Example: Page=205
- REFNO=
- Substring in literature ref ID. Example: REFNO=f92be0
- SMILES=
- Substring Example: smiles=C([SiH3])=C
- SMILES<=<SMILES>
- Substructure search. Example: smiles<=C([SiH3])=C
- SMILES~=<SMILES>
- Similarity Search. Example:smiles~=C([SiH3])=C
- Source=
- Substring in Source. eg. journal name. Example: Source=Organometallics
- Stereoinformation=true
- Marker for stereo Molecules. (Debugging help) Example: Stereoinformation=true
- Title
- Substring literature title. Example: title=study
- Volume=
- Substring literature volume. Example: Volume=42
- YEAR=
- Order by distance to year published. Example: year=2004
- date=
- Order by last edited close to date. Give as ISO date string. Example: date=2007-04-05T14:30
- error=
- Substring error messages. Example: Error=smiles
- owner
- Substring last user editing. Example: owner=me
NMR Parameters¶
- Shift=<number>
- Sort by NMR Shift values close to number. Example: shift=0.9
- Shift=<number1>...<number2>
- Shifts in range. Example: shift=0.9...0.95
- Nucleus=
- Substring in NMR Nucleus field. Example: nucleus=29Si
- Method=
- Substring in Method. Example: method=INDOR
- J=<number>
- Sort by Coubling constant closest to number. Example: j=5.4
- J=<number1>...<number2>
- Coupling Constant in range. Example: j=5.4...5.5
- jtype=
- Substring Coupling type. Example: jtype= 2J(Si-P)
- Temp=
- Substring in NMR Sample Temperature. Example: temp=-20
Design Principles¶
The single most important principle, the application tries hard not to break, is that any resource, that can be displayed, also has an URL that contains all the information required to display it again.
Also the search field is used to display the query, that led to the results. This is for example the main reason the system uses SMARTS for substructure search. Because, SMARTS encodes a chemical structure in a short string that can be part of the URL, and thus can be shown in the search field.